
Classically, this would be a state of motionlessness, but quantum uncertainty dictates that the particles still possess finite zero-point energy. This is a state at which the enthalpy and entropy of a cooled ideal gas reach its minimum value, taken as 0. This allows us to define a zero point for the thermal energy of a body.Ībsolute zero is the coldest theoretical temperature, at which the thermal motion of atoms and molecules reaches its minimum. The entropy of a system approaches a constant value as the temperature approaches absolute zero.īased on empirical evidence, this law states that the entropy of a pure crystalline substance is zero at the absolute zero of temperature, 0 K and that it is impossible using any process, no matter how idealized, to reduce the temperature of a system to absolute zero in a finite number of steps.
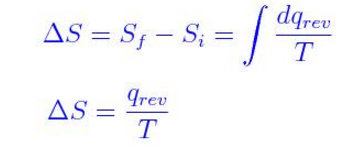
Real thermodynamic cycles have inherent energy losses due to inefficiency of compressors and turbines.Īccording to the third law of thermodynamics: Note that, the isentropic assumptions are only applicable with ideal cycles. Therefore it is very useful in power engineering because these devices are used in thermodynamic cycles of power plants. In an idealized state, compression is a pump, compression in a compressor, and expansion in a turbine is isentropic. By the definition of entropy, the heat transferred to or from a system equals the area under the T-s curve of the process.Īn isentropic process is depicted as a vertical line on a T-s diagram, whereas an isothermal process is horizontal.

The work done by or on the system and the heat added to or removed from the system can be visualized on the T-s diagram. It is used in thermodynamics to visualize changes to temperature and specific entropy during a thermodynamic process or cycle. A large number of different properties have been defined, and there are some dependencies between properties.Ī Temperature-entropy diagram ( T-s diagram) is the type of diagram most frequently used to analyze energy transfer system cycles. The phases of a substance and the relationships between its properties are most commonly shown on property diagrams. Temperature-entropy Diagrams – T-s Diagrams The absolute value of specific entropy is unknown is not a problem, however, because it is the change in specific entropy (∆s) and not the absolute value that is important in practical problems. For example, the specific entropy of water or steam is given using the reference that the specific entropy of water is zero at 0.01☌ and normal atmospheric pressure, where s = 0.00 kJ/kg. Normally, the entropy of a substance is given for some reference value. In general, specific entropy is a property of a substance, like pressure, temperature, and volume, but it cannot be measured directly. Because entropy tells so much about the usefulness of an amount of heat transferred in performing work, the steam tables include values of specific entropy (s = S/m) as part of the information tabulated.

M = mass (kg) T-s diagram of Rankine CycleĮntropy quantifies the energy of a substance that is no longer available to perform useful work. It equals the total entropy (S) divided by the total mass (m).
The specific entropy (s) of a substance is its entropy per unit mass. Engineers use the specific entropy in thermodynamic analysis more than the entropy itself. The entropy can be made into an intensive or specific variable by dividing by the mass.


 0 kommentar(er)
0 kommentar(er)
